Determine the enthalpy for the following reaction, given 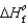 (NH3(g)) 46.1 kJ/mol,
(NH3(g)) 46.1 kJ/mol, 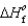 (NO(g))
(NO(g)) 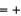 90.3 kJ/mol, and
90.3 kJ/mol, and 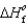 (H2O(g))
(H2O(g))  241.8 kJ/mol.
241.8 kJ/mol.
4NH3(g)  5O2(g)
5O2(g)  4NO(g)
4NO(g) 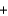 6H2O(g) Hrxn
6H2O(g) Hrxn  ? kJ
? kJ
Definitions:
Inconsistent
Lacking regularity or uniformity in thought, action, or character.
Controlled
An experiment or situation where variables are manipulated or regulated deliberately to test causality or assess outcomes.
Heider's
Fritz Heider's attribution theory focuses on how people interpret events and relate them to their thinking and behavior.
Balance Theory
A psychological theory proposing that individuals strive for psychological consistency in their relationships and beliefs, leading them to change attitudes or relationships to maintain balance.
Q8: Given the standard enthalpies of formation for
Q23: Benzoic acid is used to determine the
Q27: A compound known to contain platinum, nitrogen,
Q50: During winter in Siberia, a tenant in
Q57: Ammonium nitrate sometimes is used in cold
Q63: A compound was analyzed and found to
Q121: What is the molar mass of sulfuric
Q122: Assuming that the charge of the ions
Q146: Draw the Lewis structure for the oxalate
Q148: In the following reaction, H<sub>2</sub>O _ CH<sub>3</sub>COOH(aq)