Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction.
2N2H4(g) 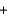 2NO2(g)
2NO2(g)  3N2(g)
3N2(g) 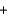 4H2O(g) Hrxn ? kJ
4H2O(g) Hrxn ? kJ
SubstanceH  in kJ/mol
in kJ/mol
N2H4(g)95.4
NO2(g)33.1
H2O(g)241.8
Definitions:
Population
The entire group of interest to researchers to which they wish to generalize their findings; the group from which a sample is selected.
Developmentally Delayed
A term used when a child does not achieve developmental milestones within the typical age range, indicating issues in physical, intellectual, or emotional growth.
Mental Ability
A measure of one's intellectual capabilities, including reasoning, problem solving, and the capacity to acquire knowledge.
Second-grade
A level of education in primary schools intended for children around seven to eight years old, focusing on basic literacy, numeracy, and introduction to other subjects.
Q9: Which of the following graphs shows the
Q25: Hypochlorous acid has the formula _<br>A)HClO<sub>4</sub>.<br>B)HClO<sub>3</sub>.<br>C)HClO<sub>2</sub>.<br>D)HClO.<br>E)H<sub>2</sub>ClO<sub>2</sub>.
Q32: Which statement about the following chemical reaction
Q48: Which of the transitions in the hydrogen
Q73: Indicate which metal requires the shortest wavelength
Q74: Plot the relationship between each of the
Q77: In going from the top to the
Q89: For a standard airbag, 131 g of
Q97: What mass of potassium iodide (molar mass
Q114: Considering that PV <font face="symbol"></font> nRT, which