Dinitrogen monoxide (N2O) is produced from nitrate ions in aqueous solution by anaerobic bacteria. Which one of the following statements about this reaction is not correct? 2NO3-(aq) 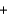 H2O(l )
H2O(l )  N2O(g)
N2O(g) 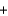 2O2(g)
2O2(g) 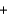 2OH-(aq)
2OH-(aq)
Definitions:
Self-disclosure
The process of revealing personal, private information to others, facilitating deeper interpersonal connections.
Personal Growth
The process of improving oneself through activities that enhance self-awareness and identity.
Lose of Identity
Refers to the experience of losing or questioning one's sense of self or identity.
Johari Window
A model used to understand and improve self-awareness, interpersonal communication, and relationships by mapping knowledge of self and others.
Q35: A temperature of 78 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="A
Q50: Commercial hydrochloric acid is 12.1 M. What
Q50: Which picture demonstrates the principle of diffusion?<br>A)
Q63: Based on its position in the periodic
Q64: What is the symbol for magnesium?<br>A)M<br>B)Mg<br>C)Mn<br>D)Mo<br>E)Ma
Q66: Some microorganisms living under anaerobic conditions extract
Q76: Which statement is correct?<br>A)Electrons, protons, and neutrons
Q84: Magnesium metal burns brightly in the air
Q125: Which stellar nuclear reaction is not correctly
Q158: The atomic radius of germanium (Z <img