When the oxidation reduction reaction shown here is balanced, how many electrons are transferred for each atom of copper that reacts? Ag+(aq) 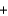 Cu(s)
Cu(s)  Ag(s)
Ag(s) 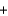 Cu2+(aq)
Cu2+(aq)
Definitions:
Ventromedial Hypothalamus
A region of the hypothalamus involved in regulating hunger and satiety, playing a key role in appetite control.
Lateral Hypothalamus
An area of the brain that plays a key role in controlling hunger and thirst.
Overeat
The action of consuming food in quantities greater than the bodily requirement, often leading to negative health outcomes.
Set Point
The weight range in which your body is programmed to function optimally and tends to stay within despite fluctuations.
Q13: Which one of the following is not
Q15: For movie night, Annabelle popped two bags
Q39: Phthalocyanine is a large molecule used in
Q66: Which statement A-D regarding an ideal gas
Q67: Diborane undergoes combustion according to the following
Q79: Medicines usually are dispensed in units of
Q93: An unknown element is found to contain
Q135: What is the symbol of the ion
Q137: If the pressure of a system is
Q160: Which transition metal ion has no d