Dinitrogen monoxide (N2O) is produced from nitrate ions in aqueous solution by anaerobic bacteria. Which one of the following statements about this reaction is not correct? 2NO3-(aq) 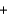 H2O(l )
H2O(l )  N2O(g)
N2O(g) 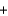 2O2(g)
2O2(g) 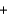 2OH-(aq)
2OH-(aq)
Definitions:
Servant Leader
A leadership philosophy in which the main goal of the leader is to serve. This is different from traditional leadership where the leader's main focus is the thriving of their company or organizations.
Altruistic Motivation
Being driven to act out of a desire to help others and improve their well-being without expecting anything in return.
Geniality
The quality of having a friendly and cheerful manner, often seen as desirable in leadership to create a positive work environment.
Servant Leader Behavior
A leadership philosophy which focuses on serving the needs of others first and foremost, with the leader prioritizing the well-being and development of their team members.
Q42: The van der Waals equation for real
Q48: The National Primary Drinking Water Regulations limit
Q77: Radiation with a wavelength of _ nm
Q79: Baking ammonia or ammonium bicarbonate (NH<sub>4</sub>HCO<sub>3</sub>, 79.1
Q88: You are given a liquid sample that
Q91: What is the temperature of CO<sub>2</sub> gas
Q132: The following graph shows the gas speed
Q136: Which statement below is not correct?<br>A)Oxygen is
Q163: What is the average kinetic energy (in
Q164: How many of these atoms or ions