Dialuminum hexachloride, Al2Cl6 (266.66 g/mol) , is an inexpensive compound that is used in many industrial processes. It is made by treating scrap aluminum with chlorine gas. If a reaction is run with 270 g of aluminum and 710 grams of chlorine, the limiting reactant is ________, and the theoretical yield is ________ of Al2Cl6. 2Al(s) 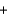 3Cl2(g)
3Cl2(g)  Al2Cl6(s)
Al2Cl6(s)
Definitions:
Atomic Masses
The mass of an atom expressed in atomic mass units, representing the total mass of protons, neutrons, and electrons in the atom.
Magnetic Field
A region around a magnetic material or a moving electric charge within which the force of magnetism acts.
Dalton's Law
The principle that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases.
Total Pressure
The sum of all partial pressures of gases in a mixture, in accordance with Dalton’s Law.
Q6: Sports trainers use cold packs containing ammonium
Q34: Which of the following is a base
Q40: Which one of the following acids has
Q47: In a steam engine, steam in a
Q49: Which one of the following is not
Q72: Motor fuels are mixtures of hydrocarbons, but
Q73: Use graphical and numerical evidence to conjecture
Q85: A bottle containing 1,665 g of sulfuric
Q122: Based on its position in the periodic
Q129: Which statement A-D about a system and