One reaction that occurs during the deployment of an air bag is shown below, where sodium metal reacts with potassium nitrate. If 5.10 g of sodium and 305 g of potassium nitrate react in an airbag, how many grams of KNO3 remain because of the limited amount of sodium present? 10Na(s) 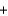 2KNO3(s)
2KNO3(s)  K2O(s)
K2O(s) 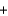 5Na2O(s)
5Na2O(s) 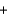 N2(g)
N2(g)
Definitions:
Credit Sales
Transactions where goods or services are sold to a customer with the agreement that payment will be made at a later date.
Zero Balance Accounts
Bank accounts that maintain a balance of zero by automatically transferring funds to cover charges or replenishing from another account.
Concentration Banking
Employing one bank to manage the balances in remote accounts. Balances are generally swept into the concentration bank daily.
Wire Transfers
Electronic transfers of funds across a network administered by banks or transfer service agencies globally.
Q3: What is the density of propane gas
Q36: Which of the following bar charts shows
Q51: Assuming that the distances between the two
Q52: On a summer day, the temperature in
Q61: The following graph shows the distribution of
Q61: The combustion of ethanol (CH<sub>3</sub>CH<sub>2</sub>OH, 46.1 g/mol)
Q63: Which of the following statements is not
Q95: What mass of potassium hydroxide is needed
Q118: Helium-neon (HeNe) lasers contain a low-pressure mixture
Q122: Assuming that the charge of the ions