Carbon monoxide is used in refining iron ore to produce the metal. Assuming the reaction yield is 100%, how many grams of iron would be produced if 5.00 mol of carbon monoxide were exposed to 800 g iron(III) oxide?
Fe2O3(s) 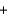 3CO(g)
3CO(g)  2Fe(s)
2Fe(s) 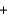 3CO2(g)
3CO2(g)
Definitions:
Stewardesses
Traditionally refers to female flight attendants, responsible for the safety and comfort of passengers aboard an aircraft.
Attractive
Qualities or features that evoke interest or desire, often used in relation to physical appearance or personality.
Single
A term often used to describe an individual who is not currently in a marital or committed romantic relationship.
Tiny Revolution
A small-scale change or shift that challenges existing norms or systems, often in a specific community or context.
Q7: Molecules are represented in various ways. Which
Q9: Find the slope of the tangent line
Q26: A significant difference in boiling points of
Q41: Elemental analysis of the soot produced by
Q85: Plot the given polar point. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg"
Q101: Find a power series representation and radius
Q115: Which statement A-D regarding the terms mole
Q117: Which one of the following statements is
Q118: Which of the following is an alkaline
Q137: Lead sinkers used for saltwater fishing do