Phosphorus trichloride reacts with water to form phosphorous acid, H3PO3, and hydrochloric acid in the following unbalanced reaction. For each mole of phosphorous acid produced by this reaction, how many moles of HCl are produced?__ PCl3 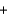 __H2O
__H2O  __H3PO3
__H3PO3 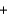 + __ HCl
+ __ HCl
Definitions:
Fixed Input
Inputs used in production that cannot be varied in the short term, such as buildings or machinery.
Plant Size
The physical capacity or dimensions of a manufacturing facility.
Implicit Costs
They represent the opportunity costs of using resources that a company already owns, rather than the out-of-pocket expenses.
Economic Costs
The total cost of production, including both explicit costs like wages and materials and implicit costs such as opportunity costs.
Q50: What is the chemical formula for calcium
Q58: Identify the acid in the following acid-base
Q64: Jupiter's mass is estimated to be 1.90
Q65: In a titration, the solution of known
Q67: Which set of gases is listed from
Q102: The diameter of the sun is 1,390,000
Q111: Sodium nitrite, which is used in meat
Q115: Which statement A-D regarding the terms mole
Q115: Zinc oxide is found in ointments for
Q140: Ammonia, NH<sub>3</sub>, is an important industrial chemical.