Calcium hydride (CaH2) is so reactive with water that it can be used to remove traces of water from solvents other than water. If 24.6 g of calcium hydride is added to a large volume of solvent that contains 14.0 g of water, which of these remains, water or calcium hydride, after the reaction is complete? The reaction is: CaH2(s) 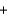 2H2O(l)
2H2O(l)  Ca(OH) 2(s)
Ca(OH) 2(s) 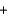 2H2(g)
2H2(g)
Definitions:
Assumptions
Premises or conditions accepted as true without proof, used as the basis for reasoning, discussion, or calculation.
Simplify
The process of making something less complex or easier to understand by reducing it to its basic components.
Scientific Method
A systematic and logical approach to discovering how things in the universe work, involving observation, hypothesis formulation, experimentation, and conclusion drawing.
Circular-flow Diagram
A model that illustrates how goods, services, and money move through an economy, depicting the interactions between households and businesses.
Q2: Write the balanced molecular equation and the
Q4: Which one of the following statements is
Q37: Which of the following is not a
Q45: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q56: Determine the radius and interval of convergence
Q58: N<sub>2</sub>O (laughing gas) is used as an
Q105: Sketch the graph of the polar equation.
Q107: Tube worms that survive near geothermal vents
Q110: In terms of the enthalpy of formation,
Q138: Chemical analysis of an organic compound found