Dialuminum hexachloride, Al2Cl6 (266.66 g/mol) , is an inexpensive compound that is used in many industrial processes. It is made by treating scrap aluminum with chlorine gas. If a reaction is run with 270 g of aluminum and 710 grams of chlorine, the limiting reactant is ________, and the theoretical yield is ________ of Al2Cl6. 2Al(s) 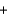 3Cl2(g)
3Cl2(g)  Al2Cl6(s)
Al2Cl6(s)
Definitions:
Abandoned Shipwreck Act
A U.S. federal law that asserts state ownership and preservation rights over abandoned shipwrecks located in its waters.
Law of Finds
A legal doctrine that governs the rights to ownership of property that has been unclaimed and discovered.
Law of Salvage
A principle in maritime law where a person who recovers another's ship or cargo after peril or loss at sea is entitled to a reward proportional to the value of the property saved.
Demolition Derby
A motorsport event where participants deliberately crash their vehicles into one another until only one is left operational.
Q5: Hartmann's solution is used in intravenous therapy
Q13: Identify the reducing agent in the following
Q15: Identify which of the following compounds are
Q29: What would you report for the total
Q34: Fe<sub>2</sub>O<sub>3</sub>(s) and powdered aluminum can react with
Q52: Graph <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Graph about
Q61: The thermite reaction is used in welding
Q94: Sulfur dioxide from coal-fired power plants combines
Q106: The carbon cycle characterizes the cyclic transformations
Q139: During a(n) _ process, energy is transferred