Like many metals, manganese reacts with halogens to give metal halides. How many grams of MnF3 can be produced using excess fluorine and 5.49 g of Mn? (molar masses: Mn = 54.9 g/mol,
F  19.0 g/mol)
19.0 g/mol)
2Mn(s) 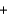 3F2(g)
3F2(g)  2MnF3(s)
2MnF3(s)
Definitions:
Present Value
The current worth of a future sum of money or stream of cash flows, given a specified rate of return.
Lease Payments
Periodic payments made by a lessee to a lessor for the use of an asset over a specified lease term.
Net Present Value
A financial metric used to evaluate the profitability of an investment, calculated by subtracting the present value of cash outflows from the present value of cash inflows.
Capital Budgeting
The process of planning and managing a firm’s investment in long-term assets.
Q9: In a self-contained breathing apparatus, potassium superoxide,
Q11: A solid forming a liquid is called
Q22: Which of the following is not a
Q27: Explain what is meant by the term
Q32: Which statement about the following chemical reaction
Q37: Assuming that the distance between ions remains
Q47: Find an equation for the indicated conic
Q49: Which graph below corresponds to the given
Q108: Identify the binary compound that has ionic
Q143: How many liters of 0.200 M NaOH