Which statement A-D about the reaction of methane with oxygen, which is called combustion and is represented by the reaction equation below, is not correct? The reaction products are carbon dioxide and water. CH4 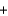 2O2
2O2  CO2
CO2 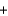 2H2O
2H2O
Definitions:
Leader Behaviours
The actions and practices displayed by a leader, which influence the motivation, performance, and well-being of their followers.
Leadership Effectiveness
The degree to which a leader successfully influences others towards achieving defined organizational or group goals.
Intelligence
The capacity to learn, understand, and apply knowledge, solve problems, and adapt to new situations.
Dominance
Exerting power or influence over others, often associated with leadership or assertiveness in social and organizational contexts.
Q19: Find an antiderivative. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Find an
Q21: Determine convergence or divergence of the series.
Q23: Which statement is not correct?<br>A)Electrons have a
Q26: Show that <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Show that
Q35: Find all polar coordinate representations of the
Q47: To bake a cake, it requires 16
Q65: Filtration can be used to separate components
Q68: Determine if <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Determine if
Q135: Determine the molar concentration of ethanol (C<sub>2</sub>H<sub>6</sub>O)
Q141: Oranges derive their distinct smell from a