Consider the following reaction at equilibrium: CO₂ + H₂O 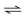 H₂CO₃. What would be the effect of adding additional H₂O?
H₂CO₃. What would be the effect of adding additional H₂O?
Definitions:
Increases Competition
A market condition where there is a rise in the number of firms or the level of competition, leading to greater choice and potentially lower prices for consumers.
Social Regulation
Laws or guidelines focused on improving health, safety, and the environment, which affect how products and services are produced, rather than price or market entry.
Natural Monopoly
A market condition in which a single firm can produce the entire market output at a lower cost than could be achieved by multiple competing firms.
Better Society
A concept or vision of a community or nation where living conditions, opportunities, and welfare are improved for all its members.
Q12: Which of the following macromolecules leave the
Q24: Thylakoids, DNA, and ribosomes are all components
Q28: The oxygen released by photosynthesis is produced
Q35: How many electrons does one atom of
Q46: A(n) _ (2 words) is a collection
Q60: Computer security focuses on protecting information, hardware,
Q62: Which of the summary statements below describes
Q65: These third-generation languages are designed to express
Q69: The executive support system can analyze the
Q72: A _ represents a collection of attributes