Figure 4-10 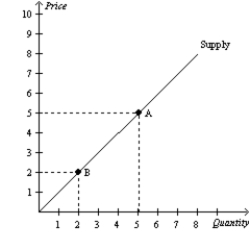
-Refer to Figure 4-10.The movement from Point A to Point B represents a(n)
Definitions:
σ Bonds
Atomic orbitals overlap linearly to form a sigma bond, providing a strong covalent bond between atoms.
Parallel P Orbitals
P orbitals that are oriented in the same spatial direction, allowing overlap and sharing of electrons between atoms, contributing to the formation of pi bonds in molecules.
Sideways
In the context of chemistry or physics, this term does not have a specific technical meaning. NO.
Carbon-Carbon
Refers to bonds between carbon atoms, fundamental in organic chemistry, forming the backbone of organic molecules.
Q48: Refer to Table 4-1. If the market
Q68: Which of the following events must cause
Q233: Refer to Figure 4-24. All else equal,
Q311: Refer to Figure 4-20. If price is
Q342: If a increase in income decreases the
Q392: If a country has the comparative advantage
Q495: Some countries win in international trade, while
Q501: The law of supply states that, other
Q511: A likely example of substitute goods for
Q613: When quantity supplied decreases at every possible