Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. Write the balanced reaction equation for the cell and report the sum of the stoichiometric coefficients. Zn(OH) 2(s) + 2e- Zn(s) + 2OH-(aq)
-1) 249 V
O2(g) + 2H2O( 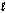 ) + 4e- 4OH-(aq)
) + 4e- 4OH-(aq)
+0) 401 V
Definitions:
Illegally Fired
Termination of employment in violation of legal protections, such as anti-discrimination laws, labor laws, or contractual agreements.
Free Speech Rights
The rights guaranteed by laws or constitutions that allow individuals to express themselves without government interference or regulation.
Free Choice Rights
The rights of employees to make their own decisions regarding union membership and representation without coercion or undue influence.
Decertification Election
A process through which employees can vote to remove the representation of a labor union in their workplace.
Q6: If the value of a common stock
Q24: Which of the following will tend to
Q25: The net asset value is the price
Q31: Methanol fuel cells depend on the following
Q37: Iodine's role is very specific. It is
Q60: Two of the units used to measure
Q78: A portfolio consists of three bonds as
Q105: Which one of the following statements concerning
Q123: In the Brønsted-Lowry definition of acids and
Q124: Liquid yield option notes or LYONS have