Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. A cell must expend energy to maintain this concentration gradient by moving ions from one side of the membrane to the other. How much energy must be expended to transport 1 mole of sodium ions across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37°C (body temperature)?
(Given: Na+ + e- a, 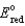 = -2.71 V; F = 96,485 C; 1 J = 1 C V; R = 8.315 J / mol K)
= -2.71 V; F = 96,485 C; 1 J = 1 C V; R = 8.315 J / mol K) 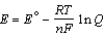
Definitions:
Chlorine Level
The concentration of chlorine present in a substance, commonly measured in water to ensure safety and hygiene.
Confidence Interval
A range of values derived from sample data that is likely to contain the true population parameter with a specified level of confidence.
Water Park
A recreational facility featuring water play areas, such as swimming pools, water slides, and splash pads.
Margin of Error
A statistic expressing the amount of random sampling error in a survey's results, indicating the range within which the true measure is expected to lie.
Q20: Compared to yields on general purpose money
Q44: Aspartame exists as a zwitterion with a
Q51: You should spend money on housing, clothing
Q70: Carbon dioxide in the atmosphere dissolves in
Q76: Which one of the following has the
Q81: The holder of a serial bond receives
Q82: What is the correct formula for sodium
Q93: Which statement, A-D, regarding the activity of
Q101: Co(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>(aq) absorbs light in the blue region
Q110: An increase in interest rates has a