It is interesting to compare the energy produced by fusion to that produced by combustion. When carbon burns to produce carbon dioxide, 395 kJ/mol of energy is released. Approximately how much greater is the energy per mole of deuterium that is released in the following fusion process: 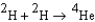 ? The atomic masses are 2.0104 g/mol for deuterium and 4.0026 g/mol for helium. Be sure to keep track of the units and get them correct; remember, 1 kJ = 1000 kg m2/s2.
? The atomic masses are 2.0104 g/mol for deuterium and 4.0026 g/mol for helium. Be sure to keep track of the units and get them correct; remember, 1 kJ = 1000 kg m2/s2.
Definitions:
Positive Accounting Theory
A theory that explains why firms elect certain accounting practices based on hypotheses about management behavior.
Ownership
The state or fact of exclusive rights and control over property, which can be an object, land/property, or intellectual property.
Control
In the context of business, control refers to the power to govern the financial and operating policies of an entity so as to obtain benefits from its activities, often achieved through ownership of more than half of the voting rights or otherwise.
Q13: Which statement regarding voltaic cells is not
Q14: Collateralized mortgage obligations are relatively low risk
Q15: The release of radioactive cesium-137 into the
Q27: Identify the Lewis acid in the following
Q29: In the financial markets, individuals are net
Q41: The molecule drawn below is an example
Q44: Aspartame exists as a zwitterion with a
Q55: Investment-grade bonds are more interest rate sensitive
Q81: How would you calculate K<sub>b</sub> for the
Q90: Convertible bonds will retain their value as