Using the following data, determine the standard cell potential 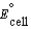 for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s)
for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s)
Half-reaction Standard Reduction Potential
Zn2+(aq) + 2e- Zn(s) -0.763
Pb2+(aq) + 2e- Pb(s) -0.126
Definitions:
Custom Cabinetry
Tailor-made cabinetry designed and built to meet specific requirements and tastes of the customer.
Product Element
A component of a product that includes features, design, quality, brand name, and packaging which together form the product's overall offer.
Marketing Mix
The set of actionable marketing tools—Product, Price, Promotion, and Place—that the firm uses to pursue its marketing objectives in the target market.
Volume Discounts
A pricing strategy where the price per unit of goods decreases as the quantity purchased increases.
Q47: Complex ions with different ligands have different
Q48: When an atom of uranium-235 captures a
Q50: Which of the following is a good
Q58: Barium is a nonessential element in the
Q60: Which of the following relationships are sufficient
Q60: How many unpaired electrons are there in
Q66: Danielle and Jonathan are in the 28%
Q68: A chemical equilibrium may be established by
Q88: Ethylenediamine is an example of a _
Q98: Pass-through securities backed by pools of auto