Using the following data, determine the standard cell potential 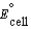 for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s)
for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s)
Half-reaction Standard Reduction Potential
Zn2+(aq) + 2e- Zn(s) -0.763
Pb2+(aq) + 2e- Pb(s) -0.126
Definitions:
Surgical Consent
Formal permission granted by a patient or caregiver for a surgical procedure, after understanding the risks and benefits.
Preoperative Education
Information and guidance provided to patients to prepare them mentally, physically, and emotionally for an upcoming surgical procedure.
Ambulatory Surgical Patient
A patient who undergoes surgery and is discharged on the same day, without the need for hospital admission.
Discharge Criteria
The specific conditions and guidelines under which a patient is considered stable and safe to leave a medical facility.
Q10: A reaction X + 2Y <font face="symbol"></font>
Q22: Values for <font face="symbol"></font>H° and <font face="symbol"></font>S°
Q41: Does pH have an effect on the
Q47: The active site of an enzyme typically
Q54: Investors can postpone or avoid income taxes
Q60: Reduction is the _<br>A) gain of electrons.<br>B)
Q62: Which of the following statements are correct
Q77: Magnesium is found in which one of
Q81: How would you calculate K<sub>b</sub> for the
Q103: An increase in the market rate of