Identify the strongest reducing agent in the following half-reactions. The standard reduction potentials are listed. -0.95 VSnO2(s) + 2H2O( 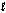 ) + 4e- Sn(s) + 4OH-(aq)
) + 4e- Sn(s) + 4OH-(aq)
+0) 61 VHg2SO4(s) + 2e- 2Hg(l) + SO42-(aq)
-1) 48 VCr(OH) 3(s) + 3e- Cr(s) + 3OH-(aq)
+1) 22 VMnO2(s) + 4H+(aq) + 2e- Mn2+(aq) + 2H2O(l)
Definitions:
Data Collected
Information gathered from various sources used for analysis, decision-making, or reporting in a wide array of contexts.
Variable Costs
Expenses that vary in relation to the amount of activity or the quantity of output produced.
Fixed Costs
Expenses that remain constant regardless of production or sales volume, including rent, salaries, and insurance costs.
Operating Income
An indication of a company's profitability from its core business operations, excluding income and expenses from unusual, non-operational activities.
Q7: A series of four equilibrium steps can
Q22: Which of the following is not a
Q33: The following energy profiles for four different
Q52: Which of the following units is used
Q53: The first disinfectant used by Joseph Lister
Q84: In the Brønsted-Lowry definition of acids and
Q89: Rhenium-186 has medical applications. It decays by
Q101: Co(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>(aq) absorbs light in the blue region
Q107: Stalactites-the long, icicle-like formations that hang from
Q139: Which of the following would be the