What is the standard cell potential for this electrochemical cell? It consists of two compartments, labeled C1 and C2.
The temperature is 298 K.
C1 consists of a copper metal electrode immersed in a 0.30 M copper sulfate solution.
C2 consists of a copper metal electrode immersed in a 1.5 M copper sulfate solution.
Cu2+ + 2e- Cu, 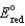 = +0.34 V
= +0.34 V
Definitions:
Central Limit Theorem
A statistical theory that states that given a sufficiently large sample size from a population with a finite level of variance, the mean of all samples from the same population will be approximately equal to the mean of the population.
Sample Mean
The average value of a sample subset of a larger population, used as an estimate of the population mean.
Point Estimate
A single value estimate for a population parameter derived from a sample.
Q10: The following figure shows Arrhenius plots for
Q11: Which of the following groups, A-D, consist
Q11: When a molecule of ethylenediamine replaces two
Q18: What is the molar concentration of silver
Q32: The charge supplied by a battery can
Q46: Which of the following is a chelation
Q72: Calculate the pH of a solution that
Q82: Sodium hypochlorite is a common ingredient in
Q98: Glucose is to cellulose as _<br>A) pure
Q132: Phenylephrine (PE, see the structure below) is