Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 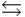 H2PO4- + H3O+
H2PO4- + H3O+ 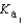 H2PO4- + H2O
H2PO4- + H2O 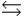 HPO42- + H3O+
HPO42- + H3O+ 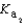 HPO42- + H2O
HPO42- + H2O 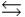 PO43- + H3O+
PO43- + H3O+ 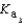 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Definitions:
Ledger
A book or collection of financial accounts in which transactions are recorded and summarized.
Net Loss
Occurs when a company's total expenses exceed its total revenues, indicating a negative profit for a specific time period.
Prepaid Insurance
An asset account that represents benefit expenses that have been paid in advance and are gradually recognized as expenses over the policy period.
Unearned Revenue
Money received by a company for goods or services yet to be delivered or provided, considered a liability until the goods or services are delivered.
Q8: Oxalic acid is a dicarboxylic acid with
Q14: Which of the following species is a
Q32: An increase in the number of moles
Q58: The degree of ionization _<br>A) increases with
Q64: Which of the following metal ions can
Q67: What repulsive forces must be overcome for
Q73: The reaction Cr(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>(aq) + 3<sup> </sup>en(aq) <font
Q88: How many different nucleic acid bases are
Q102: Which one of the following would make
Q111: Organic chemistry encompasses the chemistry of all