In the following reaction in aqueous solution, the acid reactant is __________, and its conjugate base product is __________. CH3NH2 + HSO4- 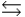 CH3NH3+ + SO42-
CH3NH3+ + SO42-
Definitions:
Boiling Point
The specific temperature at which a liquid undergoes vaporization by equating its vapor pressure with the external atmospheric pressure.
Dichlorobenzene
An organic compound consisting of a benzene ring with two chlorine atoms attached, used widely as an industrial solvent and chemical precursor.
Proton-decoupled
A technique in NMR spectroscopy where the interaction between protons is removed or decoupled, simplifying the spectrum and focusing on other interactions.
NMR Spectrum
A representation of the nuclear magnetic resonance of a molecule, providing detailed information about the structure, dynamics, reaction state, and chemical environment of molecules.
Q35: What is the pOH of a 0.20
Q37: For the reaction 2A + 3B <font
Q42: The energy profiles for four different reactions
Q48: In a rate law, the partial orders
Q51: Which of the following is true for
Q78: Which of the relationships A through D
Q84: Write the equilibrium expression for the reaction
Q90: A sketch of the free energy versus
Q116: Derive the Henderson-Hasselbalch equation from the acid
Q135: The solubility of PbBr<sub>2</sub> is 0.427 g