Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 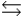 H2PO4- + H3O+ Ka
H2PO4- + H3O+ Ka
H2PO4- + H2O 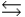 HPO42- + H3O+ Ka
HPO42- + H3O+ Ka
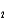 HPO42- + H2O
HPO42- + H2O 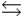 PO43- + H3O+ Ka
PO43- + H3O+ Ka
 What is the Kb expression for the base, sodium phosphate?
What is the Kb expression for the base, sodium phosphate?
Definitions:
Net Income
The final income of a firm following the deduction of all expenditures, taxes, and interest from the gross revenue.
Dividends
Payments made by a corporation to its shareholder members, usually as a distribution of profits.
Income Statement
A financial statement that shows a company's revenues and expenses over a specific period, revealing profit or loss.
Retained Earnings
Retained earnings are the portion of a company’s profits that is kept or retained rather than being paid out as dividends to shareholders or reinvested into the business.
Q14: The primary structure of a protein is
Q20: The linear form of the Arrhenius equation
Q36: Ions need to move in and out
Q39: Which sketch best represents the qualitative molecular
Q54: If the rate of the reaction: 2O<sub>3</sub>(g)
Q55: The energy profiles for four different reactions
Q88: The half-life (t<sub>1/2</sub>) of a first-order reaction
Q91: A patient is injected with a 5.0
Q125: A scientist conducts an experiment to determine
Q148: The stronger the acid, _<br>A) the stronger