The equilibrium constant for the formation of hydrogen iodide from hydrogen and iodine is 45.0 at a certain temperature. H2(g) + I2(s) 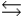 2 HI(g)
2 HI(g)
Which of the following is true regarding this equilibrium?
(I) The reaction is product favored.
(II) The reaction is reactant favored.
(III) Equilibrium lies to the right.
(IV) Equilibrium lies to the left.
Definitions:
Life Cycle
The series of stages through which a living organism passes, from its inception to the generation of new individuals.
Inguinal Hernia
A condition where soft tissue, often part of the intestine, protrudes through a weak point in the abdominal muscles near the groin area.
FLACC Tool
A behavioral scale used to assess pain in children who are unable to verbally communicate, considering factors like facial expression, leg movement, activity, cry, and consolability.
FACES Pain Rating
A visual scale for assessing pain intensity, often using faces ranging from smiling (no pain) to crying (worst pain).
Q8: The rate of disappearance of HI in
Q27: Without working out all the structures, which
Q33: In a spontaneous process, which of the
Q51: Which base is not present in RNA?<br>A)
Q59: The ordering of subunits, such as amino
Q63: In fluorite, _<br>A) cations are smaller than
Q78: Which of the listed perturbations will change
Q80: The correct name for [Co(NH<sub>3</sub>)<sub>6</sub>][Cr(CN)<sub>6</sub>] is _<br>A)
Q105: Which of these functional groups does not
Q111: The following figure shows Arrhenius plots for