The equilibrium constant for the formation of calcium carbonate from the ions in solution is 2.2 108 according to the reaction: Ca2+(aq) + CO32-(aq) 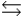 CaCO3(s)
CaCO3(s)
What is the value of the equilibrium constant for the reverse of this reaction?
Definitions:
Variable
A characteristic, number, or quantity that can change or vary and is used in scientific research to measure or test outcomes.
Hypothesis
A proposed explanation for a phenomenon made as a starting point for further investigation, testing, or experimentation.
Insider's Viewpoint
An approach or perspective that emphasizes understanding a group, culture, or situation from the point of view of its members.
Reactivity
In research, reactivity refers to the phenomenon where subjects alter their behavior or responses due to the awareness of being observed.
Q26: The pK<sub>a</sub> of hydrated iron(III) is 2.5,
Q34: Are the methyl groups in xylene included
Q40: Which of A through D is not
Q59: The mechanism for the reaction 2H<sub>2</sub>O<sub>2</sub>(aq) <font
Q71: For the rate law Rate = k[A][B]<sup>3/2</sup>,
Q74: Which of the following states of motion
Q77: Describe what is meant by the four
Q91: Which one of the following items does
Q106: As the pH decreases, the solubility of
Q120: An approximately spherical allotrope of carbon containing