The sulfide ion, S2-, reacts with water as a weak base: S2- + H2O 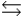 SH- + OH- K = 1.0
SH- + OH- K = 1.0
If sodium sulfide were dissolved in water to make a solution of 0.50 M, what would be the resulting concentration of OH-?
Definitions:
Petty Cash Receipt
A document used to record small, miscellaneous expenses paid with petty cash funds.
Cash Equivalents
Short-term, highly liquid investments that are easily convertible into a known amount of cash and close to their maturity.
Commercial Paper
An unsecured, short-term debt instrument issued by corporations, typically for the financing of accounts receivable, inventories, and meeting short-term liabilities.
U. S. Treasury Bills
Short-term government securities issued by the United States Department of the Treasury with maturity periods of one year or less.
Q19: A substance that can act as both
Q35: To solve an equation of the form
Q40: How many kilograms of aluminum metal can
Q45: Phosphoric acid is a triprotic acid, ionizing
Q66: A sketch of the free energy for
Q76: Which of the following will have the
Q78: Which of the listed perturbations will change
Q79: Unlike most transition metal compounds, those of
Q95: Bronze that is composed of 10% tin
Q153: A solution of the weak acid, HF,