The mechanism for the reaction 2H2O2(aq) 2H2O( 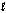 ) + O2(g) in the presence of I-(aq) is proposed to be: Step 1: H2O2(aq) + I-(aq) H2O(
) + O2(g) in the presence of I-(aq) is proposed to be: Step 1: H2O2(aq) + I-(aq) H2O( 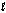 ) + OI-(aq)
) + OI-(aq)
(slow)
Step 2: H2O2(aq) + OI-(aq) H2O( 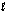 ) + O2(g) + I-(aq)
) + O2(g) + I-(aq)
(fast)
What is the molecularity of the rate-determining step?
Definitions:
Independent Variable
A variable that is manipulated or changed in an experiment to determine its effect on the dependent variable.
Room Temperature
A general description of a comfortable indoor environment, typically considered to be around 20°C (68°F) to 22°C (72°F).
Noise Level
The measurement of the ambient level of sound, often referring to the background sound present in a given environment.
Internal Validity
The degree to which a study’s results are attributable to the independent variable, rather than other factors, indicating the study accurately reflects the relationship being tested.
Q4: A saturated compound composed solely of carbon
Q12: Which is the correct formula for potassium
Q27: The rate of a reaction is found
Q31: Cylinders of NO gas may contain small
Q44: Which of the noted functional groups are
Q53: Which of the noted functional group or
Q58: Which of the following occurs when reactants
Q85: The standard cell potential for the nickel-cadmium
Q102: Which one of the following would make
Q103: What is the oxidation state of the