Multiple Choice
Determine Grxn for C4H10( 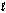 ) +
) + 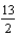 O2(g) 4CO2(g) + 5H2O(g) given the following. Substance
O2(g) 4CO2(g) + 5H2O(g) given the following. Substance
G  (J/mol · K)
(J/mol · K)
C4H10( 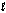 )
)
-15) 0
CO2(g)
-394.4
H2O(g)
-228.57
Definitions:
Related Questions
Q6: Which one of the following salts forms
Q27: In a titration of monoprotic acids and
Q45: For the reaction 2A + 3B <font
Q48: What is the name of [Fe(NH<sub>3</sub>)<sub>5</sub>Br]<sup>2+</sup>?
Q57: Which of the listed perturbations would change
Q71: Which of the following ions is linear?<br>A)
Q78: Which of the listed perturbations will change
Q96: For each of the bonds in the
Q101: Which of the following are true regarding
Q103: The concentration of acetic acid (pK<sub>a</sub> =