Calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: -328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction:
Na(s) + 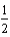 F2(g) NaF(s) ; H = -575 kJ
F2(g) NaF(s) ; H = -575 kJ
Definitions:
Time Deposit
A bank deposit with a fixed term or period of maturity, often offering higher interest rates than regular savings accounts.
Maturity
The time at which a financial obligation is due to be paid or a financial instrument, such as a bond, reaches its final installment.
Negotiable Instruments
Negotiable instruments are financial documents that promise payment to the holder and are freely transferable, such as checks, promissory notes, and bills of exchange.
Commercial Paper
An unsecured, short-term debt instrument issued by a corporation, typically for the financing of accounts receivable, inventories, and meeting short-term liabilities.
Q5: Which arrangement is in the correct order
Q13: Which of the following molecules is linear?<br>A)
Q19: Which of the following will have the
Q27: A 15.0 mg sample of a protein
Q37: Ion-dipole forces always require<br>A) an ion and
Q51: Which of the following is the shortest
Q76: The mechanism for the reaction 2H<sub>2</sub>O<sub>2</sub>(aq) <font
Q85: X-ray diffraction (XRD) makes use of the
Q92: Nylon is a _<br>A) polyester.<br>B) polyamide.<br>C) polyaminoacid.<br>D)
Q94: A sketch of the free energy for