Determine the energy change for the reaction Li(s) + 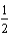 Cl2(g) LiCl(s)
Cl2(g) LiCl(s)
From the following data:
Lattice energy of LiCl = -861 kJ/mol
Energy to vaporize Li = 159 kJ/mol
Ionization energy of Li = 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: -349 kJ/mol
Definitions:
Lactate
A compound produced during anaerobic metabolism, often associated with muscle fatigue during intense exercise.
Plasma
The liquid component of blood in which blood cells are suspended, containing water, salts, and proteins.
Colloid
Atoms or molecules dispersed in a gaseous, liquid, or solid medium that resist separation from the liquid, gas, or solid.
Plasma Volume
The volume of the plasma component of blood, critical for maintaining blood pressure and supplying proteins for blood clotting and immunity.
Q15: Subshells are _<br>A) orbitals with the same
Q18: In the Bragg equation, <font face="symbol"></font> refers
Q34: In VSEPR theory, molecular geometry is determined
Q48: Where is the carbon-carbon double bond in
Q51: According to band theory, when the lower
Q54: What is the entropy change to the
Q69: Arrange the following elements in order of
Q74: Determine the molecular geometry of CF<sub>2</sub>Cl<sub>2</sub>.<br>A) linear<br>B)
Q80: Which solution will have the lowest osmotic
Q89: What mass of a 0.66 m ammonium