Calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: -328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction:
Na(s) + 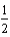 F2(g) NaF(s) ; H = -575 kJ
F2(g) NaF(s) ; H = -575 kJ
Definitions:
Fixed Manufacturing Overhead
Indirect production costs that remain constant regardless of the volume of production, such as factory rent and salaries of managers.
Direct Labour
The labor costs directly attributable to the production of goods or services, typically wages paid to workers who are directly involved in manufacturing.
Direct Materials
Raw materials that are directly traceable to the production of specific goods or services and are an integral part of the finished product.
Target Costing
A pricing strategy in which the selling price of a product is determined first, and then the manufacturing cost is managed to meet that selling price.
Q25: Which of the following statements about bonds
Q28: Which of the following photons has the
Q34: Which of the following contribute to the
Q41: Using the positions of the atoms in
Q51: According to band theory, when the lower
Q69: Arrange the following elements in order of
Q73: Astronomers have detected hydrogen atoms in interstellar
Q94: What is another name for methylbenzene?<br>A) toluene<br>B)
Q100: Which one of the following molecules or
Q115: The reaction of NO gas with H<sub>2</sub>