Determine the energy change for the reaction Li(s) + 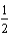 Cl2(g) LiCl(s)
Cl2(g) LiCl(s)
From the following data:
Lattice energy of LiCl = -861 kJ/mol
Energy to vaporize Li = 159 kJ/mol
Ionization energy of Li = 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: -349 kJ/mol
Definitions:
Consideration
In contract law, something of value promised, given, or received that motivates parties to enter into an agreement.
Time Draft
A written order to pay a specified amount of money at a future date.
Debtor-Creditor Relationship
A financial relationship where one party (the debtor) owes a debt to another party (the creditor).
Drawee
The drawee is the party, usually a bank, required to pay the monetary amount stated on a draft or check upon its presentation.
Q1: According to de Broglie, if the circumference
Q22: A face-centered cubic unit cell has a(n)
Q35: Which of the following compounds has a
Q37: The enthalpy of fusion for benzene is
Q41: Does the temperature of the vapors in
Q41: What type of amine is adrenaline, which
Q69: Acetonitrile (CH<sub>3</sub>CN) is an important industrial chemical.
Q70: What is the speed of an argon
Q73: What is the value of the equilibrium
Q97: In a _ reaction, the activation energy