What does the line indicated in the following phase diagram represent? 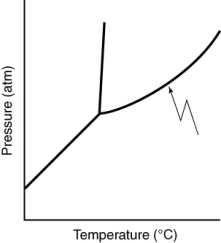
Definitions:
Equilibrium Reaction
A chemical reaction in which the forward and reverse reactions occur at the same rate, so that the concentrations of the reactants and products do not change with time.
Equilibrium Constant
A numerical value that expresses the ratio of the concentrations of products and reactants of a chemical reaction at equilibrium.
HA
Often shorthand for hydroxyapatite, a mineral form of calcium apatite; can also refer to hyaluronic acid or other context-specific chemical compounds.
Equilibrium
A state in which opposing forces or influences are balanced, often used in chemistry to describe reactions where reactants and products are formed at the same rate.
Q7: Draw the skeleton structure for 4-ethyl-3,5-dimethyl-heptane.
Q15: What functional group is found in the
Q25: The heat of fusion for water is
Q25: A 376 mg sample of a nonelectrolyte
Q27: At a historic Civil War battleground, a
Q58: If a face-centered cubic unit cell has
Q83: Which set of gases is listed from
Q86: If a mass of 100.0 g moves
Q94: Which one of the following statements regarding
Q123: Which of the following is true regarding