A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Taking into account the nonbonding electrons, identify the hybridization of the atomic orbitals for the following atoms: O1, O2, and O3. Identify the bond angles around C1, C2, and O3. 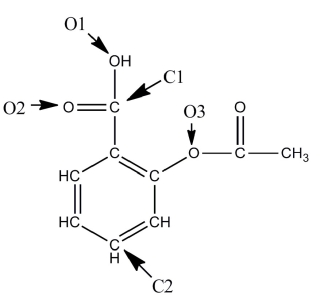
Definitions:
Males
Referring to the biological sex that produces sperm in sexual reproduction, characterized by XY chromosomes in mammals.
Hand Grip
A measure of hand and forearm strength, assessed using a device that an individual squeezes with maximum effort.
Arm Pull
A reflex in infants when an arm is pulled and the infant's hand automatically grasps the object or person as a response.
Resting Heart Rate
The number of heartbeats per minute when an individual is at rest, used as an indicator of cardiovascular fitness and overall health.
Q8: What is the hybridization of the oxygen
Q18: What is the alkane with propyl in
Q33: What <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3833/.jpg" alt="What quantum
Q45: Tube worms that survive near geothermal vents
Q50: Which of the following gases in the
Q50: Hard water contains Mg<sup>2+</sup> and Ca<sup>2+</sup> ions
Q80: A food sample was burned in a
Q82: The size of a typical atomic orbital
Q90: The monomer for polystyrene is shown below.
Q93: The formal charge on the oxygen atoms