Essay
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Taking into account the nonbonding electrons, identify the hybridization of the atomic orbitals for the following atoms: O1, O2, and O3. Identify the bond angles around C1, C2, and O3. 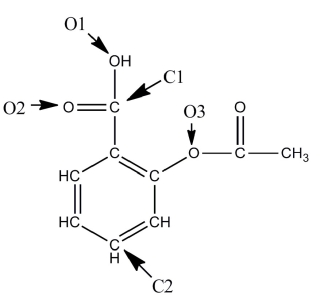
Definitions:
Related Questions
Q3: Iron crystallizes in a body-centered cubic pattern.
Q18: Which type of bonding involves the sharing
Q34: Which set of gases is listed from
Q45: Identify all of the chiral centers in
Q45: Indicate the ion that has 8 valence
Q82: Researchers at the University of Texas at
Q97: Which of the following molecules has a
Q99: Molecular orbital theory can be applied _<br>A)
Q110: Draw and name all of the isomers
Q124: Stainless steel is less susceptible to rusting