The energy of a one-electron atom is given by E = -(2.18 10-18 J) 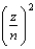 where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
Definitions:
Meaningful Result
An outcome of statistical analysis that provides significant insight or implications, often influencing decision-making or hypothesis testing.
Effect Size
A quantifiable measure of the magnitude of a phenomenon or the strength of a relationship in a statistical analysis.
Statistical Tests
Procedures used in inferential statistics to determine the probability of a hypothesis being true based on sample data.
Degrees of Freedom
The amount of distinct quantities or measures which are assignable to a statistical distribution without contravening existing constraints.
Q9: Which set of gases is listed from
Q16: Which statement about the following chemical reaction
Q27: What is the correct symbol for a
Q41: Air bags in cars inflate when an
Q45: Tube worms that survive near geothermal vents
Q60: What is the oxidation number of P
Q60: What are the stoichiometric coefficients for oxygen
Q88: Which of the following is unimportant when
Q101: Which of the following bonds is the
Q101: Calcium carbonate (100.1 g/mol) decomposes according to