The change in energy of a one-electron atom or ion for an electronic transition from the initial energy level ni to the final energy level nf is given by E = -(2.18 10-18 J) Z2 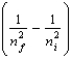 Which of the following species will have the longest wavelength emission line for the transition between the ni = 2 and nf = 1 levels?
Which of the following species will have the longest wavelength emission line for the transition between the ni = 2 and nf = 1 levels?
Definitions:
Week Number
A numerical representation of the sequential order of weeks in a calendar year, used for planning and scheduling.
Treadwear Number
A numerical rating that indicates the relative durability of a tire's tread, reflecting its expected longevity.
Rim Bead
The edge of a tire that sits tightly against the wheel rim, ensuring an airtight seal.
Valve Stems
Components that protrude from tire rims, allowing for inflation and deflation of tires.
Q13: You like boiled eggs for breakfast, but
Q14: Which of the changes, A-D, will always
Q22: The diffusion rate of H<sub>2</sub> in a
Q37: The concentration unit of molality is symbolized
Q41: Point c in the phase diagram below
Q44: Which of the following compounds would be
Q62: In addition to CO<sub>2</sub>, which of the
Q62: Draw a structure showing the geometry of
Q65: What is the symbol for magnesium?<br>A) M<br>B)
Q68: What mass of silver chloride will be