Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 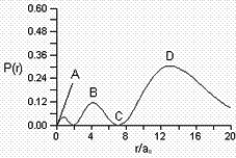 Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Definitions:
Type A Behavior
A behavior pattern characterized by aggression, competitiveness, impatience, urgency, and a strong sense of time urgency.
Individuality Corollary
A principle that stipulates individuals differ in their construction of events due to their unique experiences and perspectives.
Nonverbal Behaviors
Refers to the ways in which people communicate, intentionally or unintentionally, without using verbal language, including through gestures, facial expressions, body language, and posture.
Explanatory Style
A psychological attribute that indicates how people explain to themselves their experiences, particularly the causes of events.
Q1: Which statement regarding the fractional distillation of
Q2: The complete combustion of 2.500 g of
Q17: Nitrogen and oxygen combine to form several
Q20: Aluminum (Al) has a density of 2.70
Q39: You heat a cup of coffee in
Q66: Which arrangement is correct for increasing atomic
Q77: Iron can form two sulfides: FeS and
Q92: What is the molar mass of phosphorus
Q96: Sodium fluoride is added to drinking water
Q100: A scuba diver releases a balloon containing