The energy of a one-electron atom is given by E = -(2.18 10-18 J) 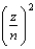 where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
Definitions:
Maker
A person or entity that creates or manufactures something.
Deposit Slip
A document provided by a bank for a depositor to fill out, detailing the amount of cash or checks to be added to a particular account.
Cryptocurrency
Digital or virtual currency that uses cryptography for security, operating independently of a central bank.
Cryptography
The practice and study of techniques for secure communication in the presence of third parties by converting information into a code to prevent unauthorized access.
Q18: What would be the products in the
Q21: Which of the following shows the orientation
Q33: What is the root-mean-square speed of N<sub>2</sub>O
Q43: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>, the local molecular
Q46: In an experiment, 30.0 g of metal
Q49: The temperature at point b in the
Q57: Which of the following diagrams shows the
Q61: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q67: Describe the difference between potential energy and
Q77: Which statement about the phase diagram below