Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 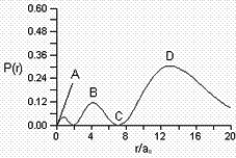 Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Definitions:
Anticipating
The act of expecting or predicting future events or needs based on current trends or data.
Background Information
Preliminary data or knowledge that provides context for a new study, discussion, or analysis.
Communication Noise
Any external or internal interference that disrupts the clarity, transmission, or reception of a message.
Interrupts
Unscheduled or unexpected disturbances that break the continuity of an activity or communication flow.
Q5: In the atoms in the Rutherford-Geiger-Marsden experiment,
Q14: Sketch a phase diagram for water and
Q17: Consider the phase diagram for a substance
Q37: The mathematical description of an electron as
Q49: How many valence electrons does S<sup>2</sup><sup>-</sup> have?<br>A)
Q53: In a chemical reaction, bonds are broken
Q61: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q62: Which of the following substances will require
Q74: Before the development of reliable batteries, miners'
Q85: Under similar conditions, which of the following