Calcium hydride (CaH2) is so reactive with water that it can be used to remove traces of water from solvents other than water. If 24.6 g of calcium hydride is added to a large volume of solvent that contains 14.0 g of water, which of these remains, water or calcium hydride, after the reaction is complete? The reaction is: CaH2(s) + 2H2O( 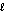 ) Ca(OH) 2(s) + 2H2(g)
) Ca(OH) 2(s) + 2H2(g)
Definitions:
Placebo Effect
A psychological phenomenon where a person experiences a perceived improvement in condition after receiving a treatment that has no therapeutic effect.
Nonalcoholic Drink
A beverage that contains no alcohol, often including soft drinks, juices, tea, coffee, and water.
Intoxicated
A state of being under the influence of alcohol or drugs, leading to impaired judgment, motor skills, and cognitive functions.
Placebo Effect
A phenomenon where a patient experiences a perceived improvement in condition due to a belief in the efficacy of a treatment that has no therapeutic value.
Q9: What is the correct symbol for an
Q9: Identify the set of stoichiometric coefficients that
Q17: Which of the following is the ground-state
Q18: The first law of thermodynamics implies that
Q21: Which of the following is a derived
Q25: What is the kinetic energy of a
Q30: This problem is from a New York
Q37: The summit of Mt. Humphreys, the highest
Q60: Which of the transitions in the following
Q87: Identify which of the following molecules has