Hydrogen peroxide (H2O2) decomposes catalytically in the presence of metal ions and enzymes called peroxidases. You may have noticed this reaction occurring if you use a drugstore solution of hydrogen peroxide as a mouthwash or antiseptic. If a 250 mL bottle of hydrogen peroxide solution containing 3.0% H2O2 (by mass) completely decomposes, how many liters of oxygen gas would it generate? Assume that 1 mol of gas occupies a volume of 22.4 L and that the density of the solution is 1.0 g/mL. The reaction is 2H2O2(aq) 2H2O( 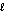 ) + O2(g)
) + O2(g)
Definitions:
Purpose
The reason for which something is done or created, or the reason for an object's existence.
Goal Setting
The practice of identifying and outlining personal or professional targets and planning steps to achieve them.
Determine Purpose
The process of identifying the specific reason or intention behind an action or task.
Attend College
The action of enrolling in and going to a tertiary education institution for pursuing higher education or a degree.
Q17: _ is/are not a consideration for the
Q25: Book value is one of the most
Q34: Write the balanced reaction equation for the
Q40: William Petty's article on harvesting notes that
Q41: It typically requires at least _ years
Q47: Which of the gas samples shown below
Q50: Traffic generators are complementary businesses that attract
Q78: Which statement regarding combustion of a sample
Q82: The most abundant metal in Earth's crust
Q93: Which statement, A-D, regarding photosynthesis is not