Calcium hydride (CaH2) is so reactive with water that it can be used to remove traces of water from solvents other than water. If 24.6 g of calcium hydride is added to a large volume of solvent that contains 14.0 g of water, which of these remains, water or calcium hydride, after the reaction is complete? The reaction is: CaH2(s) + 2H2O( 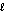 ) Ca(OH) 2(s) + 2H2(g)
) Ca(OH) 2(s) + 2H2(g)
Definitions:
Organic Product
A compound containing carbon atoms bonded to other elements such as hydrogen, often produced through organic reactions.
Structure
The arrangement of atoms or molecules in a substance that defines its chemical and physical properties.
Grignard Reagent
A class of organometallic compounds that are used in the formation of carbon-carbon bonds in chemical synthesis.
Keto Alcohol
A compound containing both a ketone (carbonyl group) and an alcohol (hydroxyl group) functional group.
Q15: Which of the following is not an
Q21: It is not necessary to have an
Q34: Which set of gases is listed from
Q37: Resources for researching the average sales per
Q40: Generic structures work well for businesses such
Q49: What pressure (in Pa) will be exerted
Q51: Hyperbaric oxygen chambers providing pure oxygen (O<sub>2</sub>)
Q53: List three reasons why the actual yield
Q71: A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3833/.jpg" alt="A atom
Q89: Molarity, M, is defined as _<br>A) moles