Calculate ΔG° for the reaction Cu(s) + H2O(g) → CuO(s) + H2(g) at 500K. 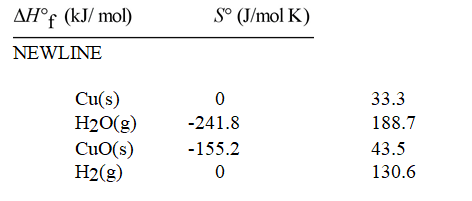
Definitions:
Internal Rate Of Return
A financial metric used to evaluate the profitability of an investment, representing the discount rate that makes the net present value of all cash flows from the investment zero.
Present Value
The value now of a future monetary sum or cash flows, considering a specified rate of return.
Desired Rate
A target interest rate that an individual or entity seeks to achieve on an investment.
Income Tax
Taxes imposed by governments on the income generated by businesses and individuals within their jurisdiction.
Q5: In which of the following one molar
Q12: Which of the following has no effect
Q19: The electron group geometry for XeF2 is
Q26: Which of the following exhibits ferromagnetism?<br>A) Ni<br>B)
Q27: Choose the INCORRECT statement.<br>A) An electrode is
Q29: Determine the [F-] of the following solution.
Q44: What is the value for Kc if
Q45: For the reaction PCl<sub>5</sub> (g) ⇌ PCl<sub>3</sub>
Q51: Choose the INCORRECT statement.<br>A) Aqua regia is
Q69: What is E° of the spontaneous cell