According to the phase diagram given, which of the following statements is INCORRECT? 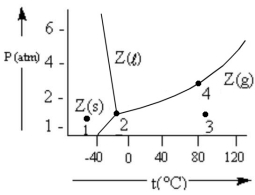
Definitions:
Nursing Interventions
Actions undertaken by nurses to benefit patients directly, ranging from medical treatment to emotional support.
Emergency Department
A specialized department in a hospital that provides immediate treatment to patients with acute illnesses or injuries.
Bruising
The discoloration and swelling of skin due to damaged blood vessels underneath, usually caused by an injury.
Police Custody
The situation where an individual is held by the police, preventing them from leaving, typically following an arrest.
Q26: Which of the following carbon molecules has
Q43: Which of the following is least likely
Q49: The reaction A + B → C
Q55: The vapor pressures of pure propyl alcohol
Q64: After drawing the Lewis dot structure for
Q72: In a Lewis structure, a terminal atom
Q76: Energy of activation has no effect on
Q80: Commercial nitric acid is 16.0 M HNO<sub>3</sub>(aq)
Q81: Which of the following are thermodynamic state
Q91: A heterogeneous catalyst is a catalyst that