According to the phase diagram given, which of the following statements is INCORRECT? 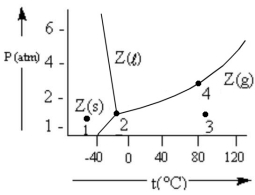
Definitions:
Medical Insurance
Health coverage that typically pays for medical, surgical, prescription drug, and sometimes dental expenses incurred by the insured.
Health Risks
Potential factors or behaviors that can lead to disease, injury, or other health problems.
Moral Hazard
A situation where one party engages in risky behavior knowing that it is protected against the risk because another party will incur the cost.
U.S. Population
The total number of residents living in the United States, as measured by census and population estimates.
Q23: For the reaction; N<sub>2</sub>(g) + 3 H<sub>2</sub>(g)
Q26: The solubility of CO in water at
Q30: Which of the following has a molecular
Q34: A liquid is in equilibrium with its
Q49: Commercial perchloric acid is 70.0% by mass,
Q52: Choose the INCORRECT statement.<br>A) Molecules with all
Q69: A crystal does not conduct electricity, even
Q86: Which statement regarding the wave function as
Q87: When an electron in an atom goes
Q97: Consider the reaction: HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub> + H<sub>2</sub>O ⇌