According to the phase diagram given, which of the following statements is wrong? 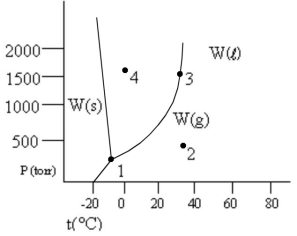
Definitions:
High-context Cultures
Cultures where communication is often indirect and relies heavily on context, non-verbal cues, and the relationships between speakers.
Explicitly
Stated clearly and in detail, leaving no room for confusion or doubt.
Essential Information
Information that is fundamental or critical to understanding a topic or making informed decisions.
Communication
The process of exchanging information, ideas, thoughts, and feelings between people through speaking, writing, or non-verbal methods.
Q1: Which of the following molecules has both
Q11: What is the pH of a 0.120
Q33: If the HCOO- ion is described using
Q43: Moles of solute per liter of solution
Q59: Molecular orbitals are formed by adding and
Q63: Arrange the following elements in order of
Q68: Can metal atoms form negative ions in
Q86: A handbook states that to prepare a
Q93: What is the molarity of a saturated
Q95: Which of the following species is paramagnetic?<br>A)