Multiple Choice
According to the phase diagram given, which of the following is INCORRECT? 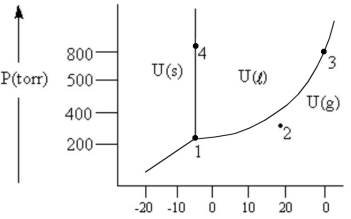
Definitions:
Related Questions
Q13: For the reaction: C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub> + 3 KI
Q14: Which of the following has a standard
Q22: Which is the correct molecular orbital diagram
Q24: In the titration of 50.0 mL of
Q45: Twenty-five milliliters of 0.10 M HCl is
Q57: Consider the reaction: CH<sub>4</sub>(g) + 4 Cl<sub>2</sub>(g)
Q61: Which of the following statements correctly describe
Q79: What is the pH of a solution
Q86: What is the pH of a 0.570
Q96: What is the pH of a solution